RTP Testing Laboratory Gets Validated By UK NEQAS
You can be assured that RTP supplies laboratory provide its clients with the highest level of quality. To that end, RTP Testing laboratory is subscribed to one of the world’s biggest External Quality Control providers, UK NEQAS, for our Covid testing.
WHY EQA
External Quality Assessment (EQA) / Proficiency Testing (PT) allows for a comparison of a laboratory’s testing procedures to other laboratories across the world. Comparisons can be made to a peer group of laboratories or to a reference laboratory. The European Centre for disease prevention and Control (ECDC), agency from the European union; encourages adoption of EU and ISO quality standards for diagnostic and reference laboratory services by its partners in EU laboratory networks and in line with national arrangements and regulations. Also the ECDC supports a large number of targeted External Quality Assessment (EQA) schemes with voluntary participation by reference or primary laboratories, which are active members of EU surveillance networks.
The rationale for these EQAs is to underpin the capability and reliability of infectious disease case detection and confirmation or agent characterisation as used for data reporting to EU surveillance and alert systems.
EQA involves running blind patient-like samples, comparing your results to peer results, in order to retrospectively monitor the accuracy of reporting. This provides confidence in the reliability of patient test results.
“EQA is defined as a system for objectively checking the laboratory’s performance using an external agency or facility.”
WORLD HEALTH ORGANISATION (2009)
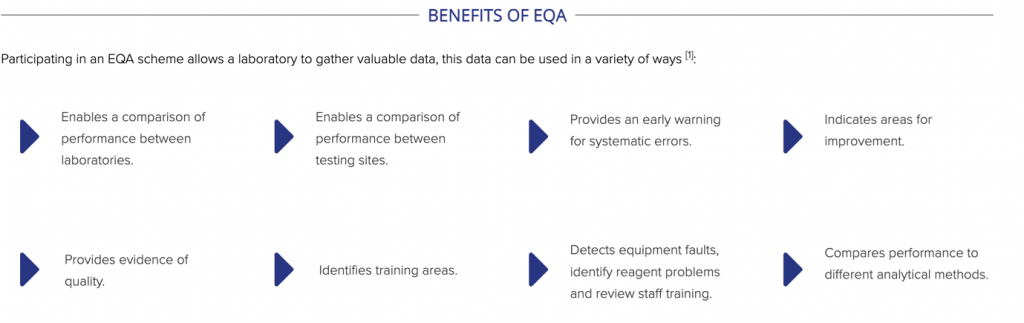
EQA provides assurance to both staff and customers that testing taking place at your laboratory provides accurate and reliable results. Problems can be identified early on and corrective action can be untaken. The reliability of methods, materials, and equipment can be evaluated and training can be developed and its impact monitored.
Why Should Members of the Public want their Laboratory to Participate in a reputable External Quality Assurance Program?
- 95% of clinical pathways relying on patients having access to pathology services
- 70% of all diagnoses relying on pathology results
- Therefore high quality pathology services form an essential part of modern healthcare
- UK NEQAS is a complete independent assurer of quality.
- UK NEQAS have been in existence for more than 50 years.
COVID-19. Brief Summary:
COVID-19, is an illness caused by SARS-CoV-2, that emerged in late 2019 and spread very quickly across the globe. Within the first two years of the COVID-19 pandemic, more than 450 million cases were reported worldwide, more than 100 million in the EU/EEA alone.
SARS-CoV-2 is mainly spread via respiratory droplets, including aerosols, from an infected person who sneezes, coughs, speaks, sings or breathes in close proximity to other people. Droplets can be inhaled or deposited in the nose and mouth or on the eyes.
Symptoms of COVID-19 can vary in severity from none at all (asymptomatic) to:
- fever
- cough
- sore throat
- general weakness, fatigue and muscle pain
- Loss of smell and taste.
The most severe cases can lead to shortness of breath due to pneumonia and acute respiratory distress syndrome, as well as other complications, potentially leading to death.
Diagnostic Techniques:
NAAT (including RT-PCR) to diagnose current infection
Types of NAATs: The diagnosis of COVID-19 is made primarily by direct detection of SARS-CoV-2 RNA by nucleic acid amplification tests (NAATs), most commonly reverse-transcription polymerase chain reaction (RT-PCR) from the upper respiratory tract. Various RT-PCR assays are used around the world; different assays amplify and detect different regions of the SARS-CoV-2 genome. Some target two or more genes, including the nucleocapsid (N), envelope (E), and spike (S) genes, and regions in the first open reading frame, including the RNA-dependent RNA polymerase (RdRp) gene. Other, less common types of NAAT include isothermal amplification, CRISPR-based assays, and next-generation sequencing. Point-of-care NAATs have also been developed, although some are less sensitive than laboratory-based tests.
Antigen testing as an alternative to NAAT
Clinical use-Tests that detect SARS-CoV-2 antigen can be performed rapidly and at the point of care and thus may be more accessible with a faster time to results than some NAATs; various home antigen tests, performed on nasal swabs, allow individuals to test themselves without presenting to medical care or a testing site. Antigen tests are typically less sensitive than NAATs, they can be useful when NAATs are not available or where NAAT turnaround times are too long to be clinically useful, provided that clinicians (and individuals who self-test) are aware of the possibility of false negatives.
RPT’s Performance in UK NEQAS for Covid testing.
Over the past 6 months, RTP testing has scored 100% in their EQA performance for SARS-CoV-2, that is for negative as well as positive results. When the result is positive for SARS-CoV-2, it is also expected of us to subculture the variant of the virus. This we have done successfully as well.
There are more than 300 laboratories across the world that participates in this program. They include some of the largest economies in the world.
